Revitalizing old blood with anti-inflammatory drug could slow aging, study suggests
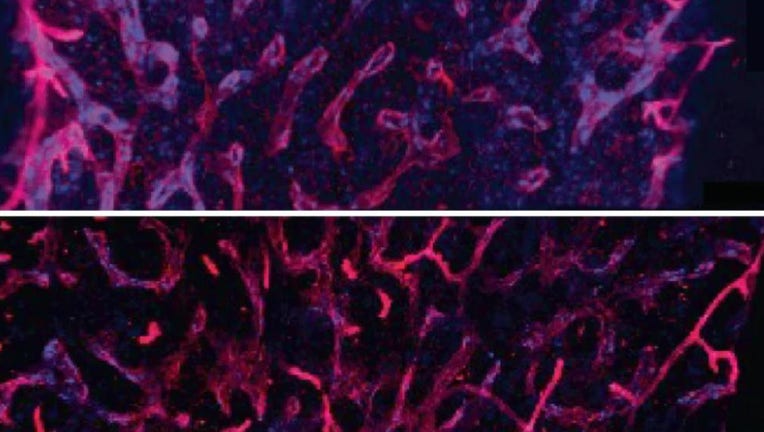
The researchers found that an inflammatory signal released from old bone marrow, IL-1B, was critical in driving aging in blood stem cells. The drug, anakinra, returned the blood stem cells to a younger, healthier state. (Images of young (top) and old
NEW YORK - Could revitalizing old blood slow the process of aging? Recent research has shown that young blood has a rejuvenating effect on aging hearts, muscles, and brains when infused into older bodies. Now, a new study suggests that an existing anti-inflammatory drug may have a similar impact without using someone else’s blood.
Researchers at Columbia University’s Stem Cell Initiative, as well as scientists from the University of Cambridge and UC San Francisco, found that the anti-inflammatory drug anakinra — already approved for use in rheumatoid arthritis — can turn back time in mice and reverse some of the effects of age on the body’s system that makes blood cells.
The results were published on Jan. 17 in Nature Cell Biology.
"An aging blood system, because it's a vector for a lot of proteins, cytokines, and cells, has a lot of bad consequences for the organism," Emmanuelle Passegué, Ph.D. and director of the Columbia Stem Cell Initiative, who's been studying how blood changes with age, said in a statement.
"A 70-year-old with a 40-year-old blood system could have a longer health span, if not a longer lifespan," Passegué added.

Could plants' DNA hold the secret to slowing the human aging process?
Scientists recently correctly identified the RNA component of telomerase in plants, and telomerase is the only enzyme capable of slowing or reversing the cellular aging process.
All blood cells in the body are created by a small number of stem cells that reside in the bone marrow, called hematopoietic stem cells, the researchers said.
Over time, these stem cells start to change: They produce fewer red blood cells, leading to anemia, and fewer immune cells, which raises the risk of infection, they said. They also start having trouble maintaining the integrity of their genomes, which can lead to blood cancers.
Passegué and her team first tried to rejuvenate old hematopoietic stem cells in mice with exercise or a calorie-restricting diet, both generally thought to slow the aging process. However, neither method worked, and transplanting old stem cells into young bone marrow also failed. Even young blood had no effect on rejuvenating old blood stem cells, the team said.
Those results were published in 2021 in the Journal of Experimental Medicine.
Passegué‘s team then took a closer look at the bone marrow, the environment or "niche" in which stem cells exist.
"Blood stem cells live in a niche; we thought what happens in this specialized local environment could be a big part of the problem," Passegué‘s graduate student Carl Mitchell said.
They found that over time, the niche deteriorates and is overwhelmed with inflammation, leading to dysfunction in the blood stem cells. The team narrowed in on one particular inflammatory signal released from the damaged bone marrow niche, called IL-1B, which was critical in driving these aging features.
They found that blocking it with the drug anakinra returned the blood stem cells to a younger, healthier state. The team said even more youthful effects on both the niche and the overall blood system occurred when the drug was used throughout the mice’s life — and not just once they were old.
Passegué‘s team is now trying to learn if the same processes are active in humans, and if rejuvenating the stem cell niche earlier in life — such as in middle age — would be a more effective strategy.
"We know that bone tissue begins to degrade when people are in their 50s. What happens in middle age? Why does the niche fail first?" Passegué said. "Only by having a deep molecular understanding will it be possible to identify approaches that can truly delay aging."

Researchers develop blood test that could help gauge depression, bipolar disorder
Researchers say they found a way to tell from a person?s blood how severe their depression is, the risk of developing severe depression in the future and the risk of future bipolar disorder.
Passegué added that treating elderly patients with anti-inflammatory drugs blocking the IL-1B function could help with maintaining healthier blood production and hopes the finding will lead to clinical testing.
Last month, scientists at Harvard University published a study that also looked at the idea of reversing aging. They found that by making DNA repairs on mice, researchers were able to drive age "forward and backward," thus manipulating the aging process.
"We believe ours is the first study to show epigenetic change as a primary driver of aging in mammals," the paper’s senior author, David Sinclair, professor of genetics at the Blavatnik Institute at Harvard Medical School and co-director of the Paul F. Glenn Center for Biology of Aging Research, said in a statement.
The researchers also acknowledged that the results must next be tested in larger mammals or humans.
This story was reported from Cincinnati.

